Credits: Albert Batalla†, Hella Janssen†, Shiral S. Gangadin and Matthijs G. Bossong († These authors contributed equally to this work.)
Source: Journal of Clinical Medicine, Published: 19 July 2019
Department of Psychiatry, UMC Utrecht Brain Center, University Medical Center Utrecht, 3508 GA Utrecht, The Netherlands, Section of Neuropsychiatry, Department of Biomedical Sciences of Cells and Systems, University Medical Center Groningen, 9713 AV Groningen, The Netherlands
Abstract:
The endogenous cannabinoid (eCB) system plays an important role in the pathophysiology of both psychotic disorders and substance use disorders (SUDs). The non-psychoactive cannabinoid compound, cannabidiol (CBD) is a highly promising tool in the treatment of both disorders. Here we review human clinical studies that investigated the ecacy of CBD treatment for schizophrenia, substance use disorders, and their comorbidity. In particular, we examined possible profiles of patients who may benefit the most from CBD treatment. CBD, either as monotherapy or added to regular antipsychotic medication, improved symptoms in patients with schizophrenia, with particularly promising effects in the early stages of illness. A potential biomarker is the level of anandamide in blood. CBD and THC mixtures showed positive effects in reducing short-term withdrawal and craving in cannabis use disorders. Studies on schizophrenia and comorbid substance use are lacking. Future studies should focus on the effects of CBD on psychotic disorders in different stages of illness, together with the effects on comorbid substance use. These studies should use standardized measures to assess cannabis use. In addition, future efforts should be taken to study the relationship between the eCB system, GABA/glutamate, and the immune system to reveal the underlying neurobiology of the effects of CBD.
1. Introduction
Schizophrenia is a complex mental disorder, which has a profound impact on patients. The burden of schizophrenia is explained by the early onset, often in early adulthood or late adolescence, its chronic course, and its relatively high prevalence [1]. The symptomatology is highly heterogeneous and often overlaps with comorbid disorders, such as affective or substance use disorders [2,3]. Psychotic symptoms are grouped into three dimensions: Positive symptoms (e.g., delusions, hallucinations), negative symptoms (e.g., blunted affect, anhedonia), and cognitive symptoms (e.g., attention, memory, executive functioning; see for reviews [4–6]). Different combinations of symptoms and comorbidity lead to different clinical profiles and treatment needs. However, the pharmacological treatment of schizophrenia is mainly based on dopamine blockade, the effect of which is limited to the positive symptoms [7]. Moreover, two-thirds of the patients experience a suboptimal response with dopaminergic treatment [8], and these results are even worse when comorbid substance use disorders (SUDs) are present [9]. Therefore, there is an urgent need for alternative and more effective pharmacological interventions aimed to reduce the burden of complex and overlapping symptom profiles.
One of these interventions may involve the endocannabinoid (eCB) system, which is a promising new pharmacological target in this respect. The eCB system consists of at least two types of receptors and their endogenous ligands (i.e., endocannabinoids; [10,11]). The cannabinoid receptors are predominantly present in the central nervous system, in particular, in several limbic and cortical brain structures [12]. The eCB system is a retrograde messenger system that regulates both excitatory glutamate and inhibitory GABA neurotransmission according to an ‘on-demand’ principle: Endocannabinoids are released when and where they are needed [10,11,13]. This endocannabinoid-mediated regulation of synaptic transmission is a widespread phenomenon in the brain and is thought to play an important role in higher brain functions, such as cognition, motor function, and processing of sensory input, reward, and emotions [14–17]. eCB receptors are also present on immune cells in the central nervous system (i.e., microglia), which suggests their involvement in processes such as cytokine release, immune suppression, and induction of both cell migration and apoptosis [18,19].
The role of the eCB system in the pathophysiology of schizophrenia has been suggested in an accumulating amount of evidence [20,21]. First, epidemiological studies suggest that cannabis use increases the risk for developing schizophrenia [22] and lowers the age of onset of the disorder [23,24]. This risk increases with a higher frequency of cannabis use (e.g., daily use), and with the consumption of more potent cannabis (i.e., a higher amount of D9-tetrahydrocannabinol; THC) [22,25–27]. Second, modulation of the eCB system by the administration of THC (i.e., the main psychoactive component in cannabis) to healthy volunteers showed that THC can induce positive psychotic symptoms, effects that resemble negative symptoms (e.g., blunted affect, lack of spontaneity) and deficits in cognition (reviewed in [28]). Importantly, in schizophrenia patients, enhanced levels of endocannabinoids were demonstrated in cerebrospinal fluid and blood [29–31], and increased CB receptor density and availability were shown in the brain [32,33].
In addition to its role in schizophrenia, there is overwhelming evidence that the eCB system is implicated in the pathophysiology of addiction, in particular in processes such as drug-seeking behaviour, reward, withdrawal, and relapse (see for reviews [34–37]). For example, animal studies have shown that addictive properties reflected in behaviours such as self-administration or conditioned place preference of opiates, nicotine, and alcohol are absent or attenuated in cannabinoid CB1-receptor knockout mice and after administration of CB1 antagonists [35]. In addition, whereas the drug seeking behaviour of drugs of abuse was blocked with CB1 antagonists, it was reinstated after the administration of CB1 agonists [34,36]. Finally, endocannabinoid concentrations are affected by active drug seeking behaviour and eCB signalling seems to modulate the rewarding effects of addictive drugs [38].
SUDs and psychotic disorders such as schizophrenia co-occur frequently. Prevalence rates of any SUD (excluding nicotine and caffeine) in patients with schizophrenia are up to 45% [39,40], with the most frequently used substances being cannabis and alcohol. Considering nicotine use disorders, the prevalence rates rise up to 60%–90% [40]. Persistent use of licit or illicit drugs has been associated with adverse consequences in the overall course of psychotic disorders, and increased morbidity and mortality [40]. In addition, SUDs are also related to poor medication adherence, increasing the risk of relapse [39]. For example, in patients with schizophrenia, cannabis use has been related to higher relapse rates, increased severity of symptoms, and poor outcome [41–45]. Despite the high co-occurring rates, patients with comorbid SUDs and psychotic disorders are often excluded from clinical trials, which limits the generalization of results and ignores the potential (positive or negative) effects of the intervention on substance use.
While THC can trigger both schizophrenia and SUD and worsen the course of both disorders, the non-psychoactive cannabinoid compound cannabidiol (CBD) may have opposite or even beneficial effects. For example, CBD may have the ability to counteract psychotic symptoms and cognitive impairment associated with cannabis use as well as with acute THC administration [46,47]. In addition,
CBD may lower the risk for developing psychosis that is related to cannabis use [48]. These effects are possibly mediated by the opposite effects of CBD and THC on brain activity patterns in key regions implicated in the pathophysiology of schizophrenia, such as the striatum, hippocampus, and prefrontal cortex [28]. Therefore, CBD displays a highly favourable profile for development as a new antipsychotic agent [48]. Similarly, CBD may serve as a treatment for SUDs, since evidence from preclinical studies suggests that CBD reduces negative withdrawal effects, motivation for self-administration, and reinstatement of drug use [37]. As a result, CBD-containing compounds are increasingly being investigated in the context of substance abuse in humans as well.
The eCB system appears an interesting target for schizophrenia, SUDs, and their comorbidity, due to the implication of the eCB system in their pathophysiology and the beneficial effects of CBD in both disorders. However, one may expect that CBD treatment may be most effective in a subgroup of patients, for example patients who show alterations in the eCB system or have a specific symptom profile. CBD may restore an imbalance in the eCB system, which may result in clinical improvement. Although previous excellent reviews (e.g., [37,48,49]) described the potential of CBD as a treatment for psychosis and SUD, this review provides a detailed and up-to-date systematic literature overview of clinical studies that investigated the ecacy of CBD treatment for schizophrenia and/or SUD. In addition, this review examined whether there are specific subgroup of patients with schizophrenia, SUD, or both that may benefit the most from CBD treatment.
2. Experimental Section
Clinical trials and case reports published up to February 2019, which described the effects of CBD on the symptomatology of psychotic disorders (i.e., schizophrenia and related disorders), SUD, or both were included. Reviews, non-English articles, pre-clinical or animal studies, studies that investigate CBD tolerability and pharmacokinetics or compare the acute effects of CBD with THC, and articles describing psychiatric or neurologic disorders other than psychotic disorders and SUD were excluded.
A literature search was conducted in the PubMed database. The following two searches were used: (1) “(cannabidiol [MeSH Terms]) OR CBD[Text Word]) AND (Substance-Related Disorders[MeSH Terms]) OR addiction[Text Word]) OR addictive behavior[Text Word]) OR drug abuse[Text Word]) OR drug dependence[Text Word])”, (2) “(Schizophrenia Spectrum and Other Psychotic Disorders[MeSH Terms])) OR schizophrenia[Text Word]) OR schizophrenic[Text Word]) OR psychosis[Text Word]) OR psychotic[Text Word])) AND ((cannabidiol[MeSH Terms]) OR CBD[Text Word])”.
3. Results
The searches resulted in 214 articles, which included one duplicate (Figure 1). The articles were screened by two authors independently, according to the PRISMA guidelines [50]. After full-text screening, ten articles from the systematic search were included and six additional papers were selected through references in other papers. Of these 16 included articles, seven studies were related to CBD treatment for schizophrenia and eight studies described the treatment of SUD with CBD-containing compounds. Only one study assessed the effects of the treatment with medicinal cannabis for patients with a psychotic disorder and a comorbid cannabis use disorder.
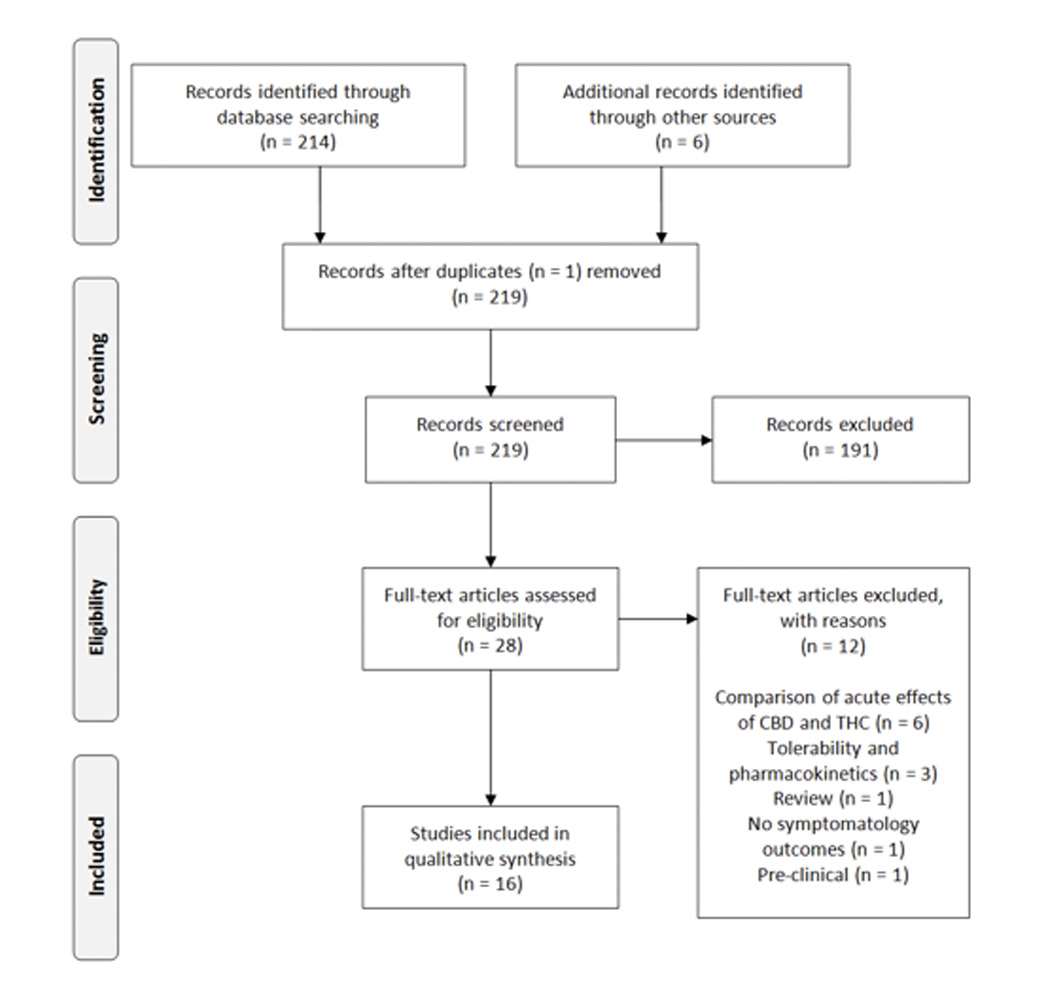
Figure 1. Study inclusion process. CBD: Cannabidiol; THC: D9-Tetrahydrocannabinol.
Four randomized controlled trials (RCTs) and three case reports assessed the ecacy of CBD as a Four randomized controlled trials (RCTs) and three case reports assessed the efficacy of CBD as treatment for psychotic disorders (Table 1). A treatment for psychotic disorders (Table 1).
Zuardi et al. (1995) described a 19-year-old woman with schizophrenia who received progressive increase of CBD monotherapy for 26 days (maximum of 1500 mg/day) [51]. CBD treatment was associated with the improvement of symptomatology as measured with the Brief Psychiatric Rating Scale (BPRS). This improvement did not further increase on haloperidol treatment [51]. In a second case report of the same group, three treatment-resistant schizophrenia male patients were treated with CBD monotherapy for four weeks. The authors reported mild improvement of positive and negative symptoms of one patient after CBD treatment (BPRS score decreased from 29 to 22). Moreover, CBD was well tolerated and no side effects were reported [52]. Makiol and Klunge (2019 described a case of a 57-year-old woman with treatment-resistant schizophrenia, which persisted for 21 years [53]. On admission she had a total PANSS (Positive and Negative Syndrome Scale) score of 117 and a negative symptom score of 41. Adjunctive to treatment with clozapine (275 mg/day) and lamotrigine (225 mg/day), the patient received CBD 500 mg twice daily, which was increased to 750 mg twice daily after seven weeks. On discharge, the PANSS total score decreased to 68 and negative symptom score to 21, which the authors indicated as accomplishment of remission criteria with only mild negative symptoms. CBD did not affect clozapine levels and was well tolerated apart from a mild hand tremor [53]. Leweke et al. (2012) performed a double-blind randomized controlled trial in which 39 acutely psychotic inpatients were treated with either CBD (N = 20) or amisulpride 800 mg (N = 19) for four weeks [54]. The authors did not provide information about illness duration before hospitalization. Both treatments were associated with clinical improvement, considering a decrease of positive and negative symptoms (change from baseline to 28-day assessment in positive PANSS score 9.0 ± 6.1 and 8.4 ± 7.5, and in negative PANSS score 9.1 ± 4.9 and 6.4 ± 6.0 after CBD and amisulpride, respectively; all comparisons p < 0.001). However, CBD treatment had a superior side-effect profile in terms of less severe changes in weight gain, extrapyramidal symptoms, prolactin levels, and sexual functioning. In addition, Leweke et al. (2012) measured anandamide levels in serum before and after treatment with CBD or amisulpride and the relationship with psychotic symptoms [54]. As compared to treatment with amisulpride, CBD showed a significant increase in anandamide levels and this was associated with the improvement of psychotic symptoms (i.e., decrease of total PANSS score). These findings suggest that anandamide levels could serve as a possible biomarker for the efficacy of CBD treatment [54]. In the largest randomized placebo-controlled trial to date, McGuire et al. (2018) assessed the effect of six-week treatment with CBD (1000 mg/day) added to antipsychotic medication in 88 moderately ill (total PANSS score >60) outpatients with schizophrenia [55]. After six weeks, positive symptoms (change from baseline 3.2 after CBD and 1.7 after placebo, treatment difference = 1.4, 95% CI = 2.5, 0.2) and global clinical impression significantly improved in the CBD group compared with placebo (treatment difference = 0.5, 95% CI = 0.8, 0.1 for improvement rates and = 0.3, 95% CI = 0.5, 0.0 for change in severity of illness) [55]. These case studies and RCTs suggest that CBD treatment for psychosis is beneficial and could possibly be as effective as antipsychotic medication.
Two RCTs showed less conclusive results of CBD treatment on positive, negative, and cognitive symptomatology. The randomized placebo-controlled trial by Hallak et al. (2010) presented the effect of acute treatment with single doses of CBD on selective attention as measured with the Stroop Colour and Word Test in a heterogeneous group of 28 schizophrenia patients (illness duration <5 years (N = 11), >5 years (N = 17)) [56]. These patients performed the Stroop test twice: The first time without the administration of any drug and the second time after oral administration of either placebo, 300 mg, or 600 mg CBD. After the two sessions, all groups showed improvement in cognitive performance (i.e., reduced number of errors during the Stroop test). Improvement was greater in the placebo and CBD 300 mg groups, compared with the patients who received CBD 600 mg. There was no effect of CBD treatment on both positive and negative symptoms [56]. Second, the most recent double-blind, randomized placebo-controlled trial by Boggs et al. (2018) examined treatment with oral CBD (600 mg/day) or placebo adjunctive to a stable dose of antipsychotic medication in 36 chronic schizophrenia patients (mean illness duration >25 years) [57]. Although positive, negative, general, and total PANSS decreased and cognitive performance increased over time in both groups, there were no significant differences between groups. Thus, in this trial, symptomatology and cognitive performance did not improve after adjunctive CBD treatment in schizophrenia outpatients who were receiving long-term polypharmacy for a myriad of psychiatric symptoms (Boggs et al., 2018) [57]. One possibility is that these results may be explained by the significant difference in the use of multiple antipsychotics between the placebo (38.9%) and CBD groups (11%) [57].
In summary, most of the abovementioned studies provided evidence for the potential of CBD as an antipsychotic treatment, which could alleviate both cognitive and psychotic symptoms in patients with psychotic disorders. The studies that showed negative results provided either a single dose of CBD [56] or included chronic schizophrenia patients who received multiple types of antipsychotic medication [57].

To date, eight studies assessed the potential effect of CBD as a treatment for SUD (Table 2). Six studies focussed on cannabis dependence and two on tobacco dependence.
3.2.1. Cannabis Dependence
The treatment of cannabis dependence with a cannabis-extracted CBD/THC mixture (Sativex) was assessed in three clinical trials. In the first double-blind randomized controlled trial by Allsop et al. (2014), 51 inpatients with cannabis dependence received Sativex or placebo for six days along with cognitive behavioural therapy [58]. Immediately after treatment, Sativex significantly decreased cannabis withdrawal and craving symptoms and improved treatment retention rates. At 28 days, both groups showed a decrease in cannabis use, in the amount of cannabis-related problems, and in the severity of cannabis dependence from baseline to follow-up, but the differences between groups were no longer significant [58]. A second double-blind randomized placebo-controlled trial assessed the effects of an eight-week treatment with self-titrated or fixed doses of Sativex in nine subjects with cannabis dependence [59]. During treatment sessions, when cannabis use was not allowed, both fixed and self-titrated doses of Sativex reduced cannabis withdrawal symptoms, however, the high fixed dose seemed the most effective. Sativex did not influence cannabis craving. The same research group performed a larger double-blind randomized placebo-controlled trial in which 27 cannabis-dependent subjects were treated with self-titrated dosages of Sativex in combination with cognitive behavioural therapy over 12 weeks [60]. The abstinence rate did not change significantly between baseline and follow-up. Cannabis use, withdrawal, and craving symptoms reduced over time in both groups. Sativex was associated with a greater reduction in cannabis craving symptoms when compared with placebo.
While the previous studies used CBD/THC mixtures, the following three studies described treatments of cannabis dependence with pure CBD. The first study by Crippa et al. (2013), reported a 19-year-old female with cannabis dependence who was treated with oral CBD over 11 days [61]. The dose was 300 mg on day 1, 600 mg on days 2–10 and 300 mg on day 11. During treatment, cannabis withdrawal, anxiety, and dissociative symptoms progressively decreased. A six-month follow-up period revealed a relapse of cannabis use, however at a lower frequency than on admission [61]. In a second case report, Shannon and Opila-Lehman (2015) described the treatment with 24–18 mg CBD oil adjunctive to citalopram and lamotrigine for a 27-year-old male with cannabis disorder and bipolar disorder. During the use of CBD oil, the patient did not use cannabis, showed a decrease in anxiety, and demonstrated improved sleep quality [62]. Third, the open-label clinical trial by Solowij et al. (2018) assessed the effects of ten-week treatment with CBD (200 mg/day) on psychological symptoms, cognition, and plasma concentrations [63]. Twenty frequent and ongoing cannabis users, of which twelve were dependent users (severity dependence scale score 3) and ten were nondependent users (severity dependence scale score <3), participated in this trial. Between baseline and post treatment sessions, cannabis use and withdrawal did not change, but cannabis-related experiences (i.e., euphoria and feeling high) decreased. Anxiety, depressive, and psychotic-like symptoms showed greater reductions in dependent than nondependent users. Attentional switching, verbal learning, and memory improved in all participants. Remarkably, higher CBD plasma concentrations were associated with lower psychotic-like symptoms (total and negative), distress, anxiety, and severity of cannabis dependence [63]. These results suggest greater effects of CBD in dependent users which can possibly be detected through CBD plasma concentrations.
Taken collectively, CBD shows some promise in the treatment of cannabis dependence as it reduces behaviour relevant to addiction such as craving and withdrawal in almost all studies. Because double-blind placebo-controlled RCTs with pure CBD are lacking, the evidence for the ecacy of products containing a combination of CBD and THC in the treatment of cannabis dependence is more convincing.
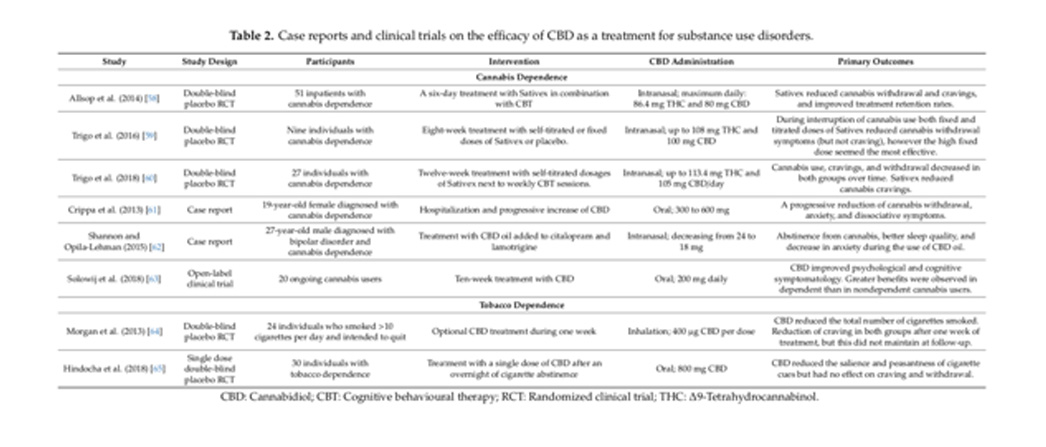
Table 2. Case reports and clinical trials on the ecacy of CBD as a treatment for substance use disorders.
Cannabis Dependence
Tobacco Dependence
CBD: Cannabidiol; CBT: Cognitive behavioural therapy; RCT: Randomized clinical trial; THC: D9-Tetrahydrocannabinol.
Morgan et al. (2013) assessed the effects of the optional use of an inhaler containing CBD (400 μg/dose) during one week in 24 individuals who smoked >10 cigarettes/day and intended to quit [64]. Results showed that CBD reduced the total number of cigarettes smoked during the treatment period. However, CBD did not have an effect on craving symptoms. In addition, craving was reduced in both groups at the end of treatment, but this did not maintain at follow-up [64]. Additionally, a second clinical trial into the ecacy of CBD treatment for tobacco dependence provided information about treatment outcomes related to motivation and evaluation. Hindocha et al. (2018) treated 30 tobacco-dependent individuals with a single dose of CBD 800 mg [65]. Attentional bias to pictorial cigarette cues was measured using a visual probe and an explicit rating task. In addition, craving, withdrawal, and side effects were assessed. After overnight cigarette abstinence, CBD reduced attentional bias to cigarette cues and pleasantness of cigarette cues, which could suggest that CBD has a potential effect on the motivational aspects of addiction. In this trial, CBD did not have an effect on craving and withdrawal. Moreover, no significant differences were found between CBD and placebo on side effects [65].
3.3. CBD—Psychosis and SUD
Schipper and colleagues (2018) were the first who described the ecacy of CBD treatment for patients with a psychotic disorder and a comorbid treatment-resistant cannabis use disorder (Table 3) [66]. Seven hospitalized patients received eight weeks of treatment with Bedrolite, medicinal cannabis that contains 0.4% THC and 9% CBD, as add-on therapy to conventional antipsychotic medication. The medicinal cannabis was supposed to substitute street cannabis used by the patients but was only provided at fixed moments during the day. Doses ranged from 0.125 to 0.5 g daily (11–45 mg CBD), depending on dose and frequency of the use of street cannabis before admission. Treatment with CBD-rich medicinal cannabis did not affect psychosis- or dependence-related symptomatology. Patients preferred street cannabis over the medicinal cannabis and started to use additional street cannabis during the treatment program [66]. The most likely explanation for these negative results was the low THC concentration in Bedrolite as compared to street cannabis. As a result, the substitution of THC-rich street cannabis by medicinal cannabis with mainly CBD may have been too abrupt for most patients.
Table 3. Studies on CBD treatment for patients with a psychotic disorder and a comorbid cannabis use disorder.
4. Discussion and Conclusions
The current review aimed to provide a detailed and up-to-date systematic literature overview of studies that investigated the efficacy of CBD treatment for schizophrenia and/or SUD. Based on this overview, a second aim was to examine whether there is a specific subgroup of patients with schizophrenia, SUD, or both that may benefit most from CBD treatment. In some but not all studies, CBD seemed effective as a treatment for psychosis and SUD. CBD may have the capacity to alleviate positive, negative, and cognitive symptoms in schizophrenia, as well as craving and withdrawal in SUD. Although most of the studies showed promising results, differences in study design, patient population, and use of concomitant medication make it difficult to define specific subgroups to whom CBD should be administered. In addition, CBD doses and administration were different between studies and most of the reviewed studies did not describe the source of CBD (i.e., synthetic or cannabis extracted), which may have different efficacy. However, the results of the reviewed studies suggested some features that may contribute to the identification of patients who may benefit most from CBD treatment.
Research into CBD treatment for psychosis provided evidence for a few possible clinical and biological characteristics of the subgroup. The effects of CBD were studied in patients in both early and later stages of psychotic disorders. Overall, acutely psychotic and early onset patients demonstrated reductions of positive and negative symptoms [51,54], while treatment-resistant and chronic patients showed less promising improvement [56,57]. Even though Makiol and Kluge (2019) described a chronic schizophrenia patient who exhibited great clinical improvement (i.e., change of total PANSS score: 49) [53], the majority of the results suggest that CBD may be more effective in the early stage of psychotic disorders. This is in line with previous studies suggesting that immune dysregulation (i.e., microglial activation) is mainly involved in the early stage of psychotic disorders [67,68]. As cannabinoid receptors are also present on microglia, it is possible that CBD exerts its effects by decreasing microglial activity [69]. Furthermore, anandamide levels in serum could serve as a possible biomarker for the ecacy of CBD treatment. For instance, Leweke et al. (2012) reported a significant increase in anandamide levels after CBD treatment, which was associated with the improvement of psychotic symptoms (i.e., decrease of total PANSS score) [54]. This is in concurrence with a previously reported inverse association between elevated anandamide levels in cerebrospinal fluid and psychotic symptoms in antipsychotic-naïve patients [29–31].
Research into CBD treatment for SUD primarily focussed on cannabis dependence. Taken collectively, CBD shows promise in the treatment of cannabis dependence as it reduces craving and withdrawal in almost all studies. However, these studies have heterogeneous study designs and administration methods. The differences in administration and dosages may provide a possible explanation for the different results observed in the included studies. For instance, THC/CBD mixtures might be more effective in reducing some features of cannabis dependence (i.e., craving, use and withdrawal) than pure CBD. Moreover, the level of cannabis dependence and intrinsic motivation for treatment, may help to define a possible subgroup of patients in which CBD is more effective. Dependent users (i.e., those with a severity dependence scale score 3), showed reduced anxiety, depression, and psychotic-like symptoms after a 10-week treatment with CBD, compared with nondependent users [63]. However, studies that include individuals with more symptoms at baseline can show greater reductions after treatment. Therefore, it is difficult to determine whether symptom severity is truly a patient characteristic that could predict better outcomes after CBD treatment. Solowij et al. (2018) also found that cannabis-related experiences decreased after treatment [63], which is in accordance with previous studies that indicate that CBD counteracts the effects induced by THC [46,47]. Intrinsic motivation for treatment seems an important aspect as well, as it may increase medication adherence [65]. Conversely, patients that do not seek treatment are less inclined to follow strict study protocols [66]. The majority of the discussed studies recruited individuals with cannabis dependence from the community, which suggests that these individuals were at least open for treatment. To a certain extent, this may explain why the study by Schipper et al. (2018) found that CBD administration was not effective [66], as they included individuals that did not seek treatment.
Considering the ecacy of CBD in both psychotic disorders and SUD, one can speculate that CBD should also be effective in the treatment of the comorbidity. However, only Schipper et al. (2018) studied this population, with negative results [66]. As discussed previously, these patients were treatment-resistant for SUD and showed lack of motivation for treatment. An additional limitation of this study was the good baseline functioning in five out of seven patients. Moreover, this study administered CBD in a formulation that contained very little THC, which possibly explains why the participants preferred street cannabis.
Future studies could take these limitations into account and should focus on examining the effects of CBD in the different stages of psychotic disorders, considering the high prevalence of comorbid SUD. Studies into psychotic disorders could use CBD (i.e., either as monotherapy or add-on) to treat psychotic symptoms and to prevent relapse in early stages, while exploring the effects on comorbid substance use (e.g., cannabis). These studies should use standardized measures to assess cannabis use. In later stages and comorbid treatment-resistant SUD, CBD studies may aim to reduce cannabis use, using harm-reduction strategies (e.g., gradually shift the THC/CBD ratio in medicinal cannabis in favour of CBD) [70]. Currently, nine ongoing clinical trials that study the effects of CBD on psychotic disorders or SUD (including alcohol and cocaine misuse) are registered in clinicaltrials.gov, of which one (NCT03883360) includes patients with recent-onset psychotic disorder and cannabis use. Therefore, more results on this topic are expected in the near future.
It remains unclear if the ecacy of CBD in schizophrenia, addiction, and their comorbidity could be explained by shared or different biological mechanisms. To elucidate this, future efforts should be taken to study the relationship between the eCB system, GABA/glutamate, and the immune system. For example, neuroimaging studies (e.g., positron-emission tomography, PET and magnetic resonance spectroscopy, MRS) could measure CB1 receptor densities and markers for glia in patients with schizophrenia and/or SUD who were treated with CBD.
In conclusion, CBD treatment is a promising and novel tool with several potential applications in the treatment of psychotic disorders, substance use disorders, and their comorbidity. Large-scale trials are needed to establish its clinical utility.
Author Contributions: Conceptualization, A.B. and M.G.B.; methodology, H.J. and S.S.G.; investigation, H.J.; data curation, H.J. and S.S.G.; Writing—Original draft preparation, A.B., H.J. and S.S.G.; Writing—Review and editing, A.B. and M.G.B.; visualization, H.J. and S.S.G.; supervision, A.B. and M.G.B.
Funding: M. Bossong was supported by a Veni fellowship from the Netherlands Organization for Scientific Research (grant number 016.166.038).
- Conflicts of Interest: The authors declare no conflict of interest. References
Rössler, W.; Joachim Salize, H.; van Os, J.; Riecher-Rössler, A. Size of burden of schizophrenia and psychotic disorders. Eur. Neuropsychopharmacol. 2005, 15, 399–409. [CrossRef] [PubMed]
- Buckley, P.F.; Miller, B.J.; Lehrer, D.S.; Castle, D.J. Psychiatric Comorbidities and Schizophrenia. Schizophr. Bull. 2009, 35, 383–402. [CrossRef] [PubMed]
- Volkow, N.D. Substance Use Disorders in Schizophrenia—Clinical Implications of Comorbidity. Schizophr. Bull. 2009, 35, 469–472. [CrossRef] [PubMed]
- Fioravanti, M.; Carlone, O.; Vitale, B.; Cinti, M.E.; Clare, L. A Meta-Analysis of Cognitive Deficits in Adults with a Diagnosis of Schizophrenia. Neuropsychol. Rev. 2005, 15, 73–95. [CrossRef] [PubMed]
- Tandon, R.; Nasrallah, H.A.; Keshavan, M.S. Schizophrenia, “just the facts” 4. Clinical features and conceptualization. Schizophr. Res. 2009, 110, 1–23. [CrossRef] [PubMed]
- Van Os, J.; Kenis, G.; Rutten, B.P.F. The environment and schizophrenia. Nature 2010, 468, 203–212. [CrossRef] [PubMed]
- Tandon, R. Antipsychotics in the Treatment of Schizophrenia. J. Clin. Psychiatry 2011, 72. [CrossRef]
- Samara, M.T.; Nikolakopoulou, A.; Salanti, G.; Leucht, S. How Many Patients with Schizophrenia Do Not Respond to Antipsychotic Drugs in the Short Term? An Analysis Based on Individual Patient Data from
Randomized Controlled Trials. Schizophr. Bull. 2019, 45, 639–646. [CrossRef]
- Green, A.I. Schizophrenia and comorbid substance use disorder: Effects of antipsychotics. J. Clin. Psychiatry
2005, 66 (Suppl. 6), 21–26.
- Kano, M.; Ohno-Shosaku, T.; Hashimotodani, Y.; Uchigashima, M.; Watanabe, M. Endocannabinoid-Mediated
Control of Synaptic Transmission. Physiol. Rev. 2009, 89, 309–380. [CrossRef]
- Katona, I.; Freund, T.F. Multiple Functions of Endocannabinoid Signaling in the Brain. Annu. Rev. Neurosci.
2012, 35, 529–558. [CrossRef]
- Wong, D.F.; Kuwabara, H.; Horti, A.G.; Raymont, V.; Brasic, J.; Guevara, M.; Ye, W.; Dannals, R.F.; Ravert, H.T.;
Nandi, A.; et al. Quantification of cerebral cannabinoid receptors subtype 1 (CB1) in healthy subjects and schizophrenia by the novel PET radioligand [11C]OMAR. Neuroimage 2010, 52, 1505–1513. [CrossRef]
- Heifets, B.D.; Castillo, P.E. Endocannabinoid Signaling and Long-Term Synaptic Plasticity. Annu. Rev. Physiol. 2009, 71, 283–306. [CrossRef]
- Hill, M.N.; Hillard, C.J.; Bambico, F.R.; Patel, S.; Gorzalka, B.B.; Gobbi, G. The Therapeutic Potential of the Endocannabinoid System for the Development of a Novel Class of Antidepressants. Trends Pharmacol. Sci. 2009, 30, 484–493. [CrossRef]
- Zanettini, C. Effects of endocannabinoid system modulation on cognitive and emotional behavior. Front. Behav. Neurosci. 2011, 5. [CrossRef]
- Bossong, M.G.; Jager, G.; Bhattacharyya, S.; Allen, P. Acute and non-acute effects of cannabis on human memory function: A critical review of neuroimaging studies. Curr. Pharm. Des. 2014, 20, 2114–2125. [CrossRef]
- Bossong, M.G.; Jansma, J.M.; Bhattacharyya, S.; Ramsey, N.F. Role of the endocannabinoid system in brain functions relevant for schizophrenia: An overview of human challenge studies with cannabis or D9-tetrahydrocannabinol (THC). Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 52, 53–69. [CrossRef]
- Pertwee, R.G. Ligands that target cannabinoid receptors in the brain: From THC to anandamide and beyond. Addict. Biol. 2008, 13, 147–159. [CrossRef]
- Cabral, G.A.; Grin-Thomas, L. Emerging role of the cannabinoid receptor CB2 in immune regulation: Therapeutic prospects for neuroinflammation. Expert Rev. Mol. Med. 2009, 11, e3. [CrossRef]
- Leweke, F.M.; Koethe, D. Cannabis and psychiatric disorders: It is not only addiction. Addict. Biol. 2008, 13, 264–275. [CrossRef]
- Bossong, M.G.; Niesink, R.J.M. Adolescent brain maturation, the endogenous cannabinoid system and the neurobiology of cannabis-induced schizophrenia. Prog. Neurobiol. 2010, 92, 370–385. [CrossRef]
- Marconi, A.; Di Forti, M.; Lewis, C.M.; Murray, R.M.; Vassos, E. Meta-analysis of the Association Between the Level of Cannabis Use and Risk of Psychosis. Schizophr. Bull. 2016, 42, 1262–1269. [CrossRef]
- Large, M.; Sharma, S.; Compton, M.T.; Slade, T.; Nielssen, O. Cannabis Use and Earlier Onset of Psychosis. Arch. Gen. Psychiatry 2011, 68, 555. [CrossRef]
- Di Forti, M.; Sallis, H.; Allegri, F.; Trotta, A.; Ferraro, L.; Stilo, S.A.; Marconi, A.; La Cascia, C.; Reis Marques, T.; Pariante, C.; et al. Daily Use, Especially of High-Potency Cannabis, Drives the Earlier Onset of Psychosis in Cannabis Users. Schizophr. Bull. 2014, 40, 1509–1517. [CrossRef]
- Schubart, C.D.; van Gastel, W.A.; Breetvelt, E.J.; Beetz, S.L.; Ophoff, R.A.; Sommer, I.E.C.; Kahn, R.S.; Boks, M.P.M. Cannabis use at a young age is associated with psychotic experiences. Psychol. Med. 2011, 41, 1301–1310. [CrossRef]
- Di Forti, M.; Marconi, A.; Carra, E.; Fraietta, S.; Trotta, A.; Bonomo, M.; Bianconi, F.; Gardner-Sood, P.; O’Connor, J.; Russo, M.; et al. Proportion of patients in south London with first-episode psychosis attributable to use of high potency cannabis: A case-control study. Lancet Psychiatry 2015, 2, 233–238. [CrossRef]
- Di Forti, M.; Quattrone, D.; Freeman, T.P.; Tripoli, G.; Gayer-Anderson, C.; Quigley, H.; Rodriguez, V.; Jongsma, H.E.; Ferraro, L.; La Cascia, C.; et al. The contribution of cannabis use to variation in the incidence of psychotic disorder across Europe (EU-GEI): A multicentre case-control study. Lancet Psychiatry 2019, 6, 427–436. [CrossRef]
- Murray, R.M.; Englund, A.; Abi-Dargham, A.; Lewis, D.A.; Di Forti, M.; Davies, C.; Sherif, M.; McGuire, P.; D’Souza, D.C. Cannabis-associated psychosis: Neural substrate and clinical impact. Neuropharmacology 2017, 124, 89–104. [CrossRef]
- Leweke, F.; Giuffrida, A.; Wurster, U.; Emrich, H.M.; Piomelli, D. Elevated endogenous cannabinoids in schizophrenia. Neuroreport 1999, 10, 1665–1669. [CrossRef]
- Giuffrida, A.; Leweke, F.M.; Gerth, C.W.; Schreiber, D.; Koethe, D.; Faulhaber, J.; Klosterkötter, J.; Piomelli, D. Cerebrospinal Anandamide Levels are Elevated in Acute Schizophrenia and are Inversely Correlated with Psychotic Symptoms. Neuropsychopharmacology 2004, 29, 2108–2114. [CrossRef]
- Leweke, F.M.; Giuffrida, A.; Koethe, D.; Schreiber, D.; Nolden, B.M.; Kranaster, L.; Neatby, M.A.; Schneider, M.; Gerth, C.W.; Hellmich, M.; et al. Anandamide levels in cerebrospinal fluid of first-episode schizophrenic patients: Impact of cannabis use. Schizophr. Res. 2007, 94, 29–36. [CrossRef]
- Ceccarini, J.; De Hert, M.; Van Winkel, R.; Peuskens, J.; Bormans, G.; Kranaster, L.; Enning, F.; Koethe, D.; Leweke, F.M.; Van Laere, K. Increased ventral striatal CB1 receptor binding is related to negative symptoms in drug-free patients with schizophrenia. Neuroimage 2013, 79, 304–312. [CrossRef]
- Ranganathan, M.; Cortes-Briones, J.; Radhakrishnan, R.; Thurnauer, H.; Planeta, B.; Skosnik, P.; Gao, H.; Labaree, D.; Neumeister, A.; Pittman, B.; et al. Reduced Brain Cannabinoid Receptor Availability in Schizophrenia. Biol. Psychiatry 2016, 79, 997–1005. [CrossRef]
- De Vries, T.J.; Schoffelmeer, A.N.M. Cannabinoid CB1 receptors control conditioned drug seeking. Trends Pharmacol. Sci. 2005, 26, 420–426. [CrossRef]
- Maldonado, R.; Valverde, O.; Berrendero, F. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci. 2006, 29, 225–232. [CrossRef]
- Fattore, L.; Fadda, P.; Spano, M.S.; Pistis, M.; Fratta, W. Neurobiological mechanisms of cannabinoid addiction. Mol. Cell. Endocrinol. 2008, 286, S97–S107. [CrossRef]
- Chye, Y.; Christensen, E.; Solowij, N.; Yücel, M. The Endocannabinoid System and Cannabidiol’s Promise for the Treatment of Substance Use Disorder. Front. Psychiatry 2019, 10. [CrossRef]
- Parsons, L.H.; Hurd, Y.L. Endocannabinoid signalling in reward and addiction. Nat. Rev. Neurosci. 2015, 16, 579–594. [CrossRef]
- Hunt, G. Medication compliance and comorbid substance abuse in schizophrenia: Impact on community survival 4 years after a relapse. Schizophr. Res. 2002, 54, 253–264. [CrossRef]
- Crockford, D.; Addington, D. Canadian Schizophrenia Guidelines: Schizophrenia and Other Psychotic Disorders with Coexisting Substance Use Disorders. Can. J. Psychiatry 2017, 62, 624–634. [CrossRef]
- Schoeler, T.; Petros, N.; Di Forti, M.; Klamerus, E.; Foglia, E.; Murray, R.; Bhattacharyya, S. Poor medication adherence and risk of relapse associated with continued cannabis use in patients with first-episode psychosis: A prospective analysis. Lancet Psychiatry 2017, 4, 627–633. [CrossRef]
- D’Souza, D.C.; Abi-Saab, W.M.; Madonick, S.; Forselius-Bielen, K.; Doersch, A.; Braley, G.; Gueorguieva, R.; Cooper, T.B.; Krystal, J.H. Delta-9-tetrahydrocannabinol effects in schizophrenia: Implications for cognition, psychosis, and addiction. Biol. Psychiatry 2005, 57, 594–608. [CrossRef]
- Foti, D.J.; Kotov, R.; Guey, L.T.; Bromet, E.J. Cannabis use and the course of schizophrenia: 10-year follow-up after first hospitalization. Am. J. Psychiatry 2010, 167, 987–993. [CrossRef]
- Batalla, A.; Bhattacharyya, S.; Yücel, M.; Fusar-Poli, P.; Crippa, J.A.; Nogué, S.; Torrens, M.; Pujol, J.; Farré, M.; Martin-Santos, R. Structural and Functional Imaging Studies in Chronic Cannabis Users: A Systematic Review of Adolescent and Adult Findings. PLoS ONE 2013, 8, e55821. [CrossRef]
- Foglia, E.; Schoeler, T.; Klamerus, E.; Morgan, K.; Bhattacharyya, S. Cannabis use and adherence to antipsychotic medication: A systematic review and meta-analysis. Psychol. Med. 2017, 47, 1691–1705. [CrossRef]
- Zuardi, A.W.; Shirakawa, I.; Finkelfarb, E.; Karniol, I.G. Action of cannabidiol on the anxiety and other effects produced by 9-THC in normal subjects. Psychopharmacology (Berl.) 1982, 76, 245–250. [CrossRef]
- Englund, A.; Morrison, P.D.; Nottage, J.; Hague, D.; Kane, F.; Bonaccorso, S.; Stone, J.M.; Reichenberg, A.; Brenneisen, R.; Holt, D.; et al. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J. Psychopharmacol. 2013, 27, 19–27. [CrossRef]
- Iseger, T.A.; Bossong, M.G. A systematic review of the antipsychotic properties of cannabidiol in humans. Schizophr. Res. 2015, 162, 153–161. [CrossRef]
- Leweke, F.M.; Mueller, J.K.; Lange, B.; Rohleder, C. Therapeutic Potential of Cannabinoids in Psychosis. Biol. Psychiatry 2016, 79, 604–612. [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [CrossRef]
- Zuardi, A.W.; Morais, S.L.; Guimarães, F.S.; Mechoulam, R. Antipsychotic effect of cannabidiol. J. Clin. Psychiatry 1995, 56, 485–486.
- Zuardi, A.W.; Hallak, J.E.C.; Dursun, S.M.; Morais, S.L.; Sanches, R.F.; Musty, R.E.; Crippa, J.A.S. Cannabidiol monotherapy for treatment-resistant schizophrenia. J. Psychopharmacol. 2006, 20, 683–686. [CrossRef]
- Makiol, C.; Kluge, M. Remission of severe, treatment-resistant schizophrenia following adjunctive cannabidiol.
Aust. New Zeal. J. Psychiatry 2019, 53, 262. [CrossRef]
- Leweke, F.M.; Piomelli, D.; Pahlisch, F.; Muhl, D.; Gerth, C.W.; Hoyer, C.; Klosterkötter, J.; Hellmich, M.;
Koethe, D. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl. Psychiatry 2012, 2, e94. [CrossRef]
- McGuire, P.; Robson, P.; Cubala, W.J.; Vasile, D.; Morrison, P.D.; Barron, R.; Taylor, A.; Wright, S. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: A multicenter randomized controlled trial. Am. J. Psychiatry 2018, 175, 225–231. [CrossRef]
- Hallak, J.E.C.; Machado-de-Sousa, J.P.; Crippa, J.A.S.; Sanches, R.F.; Trzesniak, C.; Chaves, C.; Bernardo, S.A.; Regalo, S.C.; Zuardi, A.W. Performance of schizophrenic patients in the Stroop Color Word Test and electrodermal responsiveness after acute administration of cannabidiol (CBD). Rev. Bras. Psiquiatr. 2010, 32, 56–61. [CrossRef] [PubMed]
- Boggs, D.L.; Surti, T.; Gupta, A.; Gupta, S.; Niciu, M.; Pittman, B.; Schnakenberg Martin, A.M.; Thurnauer, H.; Davies, A.; D’Souza, D.C.; et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology (Berl.) 2018, 235, 1923–1932. [CrossRef] [PubMed]
- Allsop, D.J.; Copeland, J.; Lintzeris, N.; Dunlop, A.J.; Montebello, M.; Sadler, C.; Rivas, G.R.; Holland, R.M.; Muhleisen, P.; Norberg, M.M.; et al. Nabiximols as an agonist replacement therapy during cannabis withdrawal:A randomized clinical trial. JAMA Psychiatry 2014, 71, 281–291. [CrossRef] [PubMed]
- Trigo, J.M.; Soliman, A.; Staios, G.; Quilty, L.; Fischer, B.; George, T.P.; Rehm, J.; Selby, P.; Barnes, A.J.; Huestis, M.A.; et al. Sativex associated with behavioral-relapse prevention strategy as treatment for cannabis dependence: A case series. J. Addict. Med. 2016, 10, 274–279. [CrossRef] [PubMed]
- Trigo, J.M.; Soliman, A.; Quilty, L.C.; Fischer, B.; Rehm, J.; Selby, P.; Barnes, A.J.; Huestis, M.A.; George, T.P.; Streiner, D.L.; et al. Nabiximols combined with motivational enhancement/cognitive behavioral therapy for the treatment of cannabis dependence: A pilot randomized clinical trial. PLoS ONE 2018, 13, 1–21. [CrossRef] [PubMed]
- Crippa, J.A.S.; Hallak, J.E.C.; Machado-De-Sousa, J.P.; Queiroz, R.H.C.; Bergamaschi, M.; Chagas, M.H.N.; Zuardi, A.W. Cannabidiol for the treatment of cannabis withdrawal syndrome: A case report. J. Clin. Pharm. Ther. 2013, 38, 162–164. [CrossRef]
- Shannon, S.; Opila-Lehman, J. Cannabidiol Oil for Decreasing Addictive Use of Marijuana: A Case Report. Integr. Med. (Encinitas) 2015, 14, 31–35.
- Solowij, N.; Broyd, S.J.; Beale, C.; Prick, J.-A.; Greenwood, L.; van Hell, H.; Suo, C.; Galettis, P.; Pai, N.; Fu, S.; et al. Therapeutic Effects of Prolonged Cannabidiol Treatment on Psychological Symptoms and Cognitive Function in Regular Cannabis Users: A Pragmatic Open-Label Clinical Trial. Cannabis Cannabinoid Res. 2018, 3, 21–34. [CrossRef]
- Morgan, C.J.A.; Das, R.K.; Joye, A.; Curran, H.V.; Kamboj, S.K. Cannabidiol reduces cigarette consumption in tobacco smokers: Preliminary findings. Addict. Behav. 2013, 38, 2433–2436. [CrossRef]
- Hindocha, C.; Freeman, T.P.; Grabski, M.; Stroud, J.B.; Crudgington, H.; Davies, A.C.; Das, R.K.; Lawn, W.; Morgan, C.J.A.; Curran, H.V. Cannabidiol reverses attentional bias to cigarette cues in a human experimental model of tobacco withdrawal. Addiction 2018, 113, 1696–1705. [CrossRef]
- Schipper, R.; Dekker, M.; de Haan, L.; van den Brink, W. Medicinal cannabis (Bedrolite) substitution therapy in inpatients with a psychotic disorder and a comorbid cannabis use disorder: A case series. J. Psychopharmacol. 2018, 32, 353–356. [CrossRef]
- Monji, A.; Kato, T.; Kanba, S. Cytokines and schizophrenia: Microglia hypothesis of schizophrenia. Psychiatry Clin. Neurosci. 2009, 63, 257–265. [CrossRef]
- Gangadin, S.S.; Nasib, L.G.; Sommer, I.E.C.; Mandl, R.C.W. MRI investigation of immune dysregulation in schizophrenia. Curr. Opin. Psychiatry 2019, 32, 164–169. [CrossRef]
- Gomes, F.V.; Llorente, R.; Del Bel, E.A.; Viveros, M.-P.; López-Gallardo, M.; Guimarães, F.S. Decreased glial reactivity could be involved in the antipsychotic-like effect of cannabidiol. Schizophr. Res. 2015, 164, 155–163. [CrossRef]
- Englund, A.; Freeman, T.P.; Murray, R.M.; McGuire, P. Can we make cannabis safer? Lancet Psychiatry 2017, 4, 643–648. [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Original Research Paper





